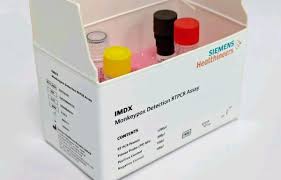
A major accomplishment for Siemens Healthineers was receiving permission from the Central Drugs Standard Control Organization (CDSCO) to provide an RT-PCR kit for the detection of monkeypox. This discovery represents a significant step forward in the fight against the ongoing public health problem that is monkeypox, or M-pox.
The recently authorized kit, called the IMDX Monkeypox Detection RT-PCR Assay, will be manufactured at the molecular diagnostics facility of Siemens Healthineers in Vadodara, Gujarat. With its enormous manufacturing capacity, this facility can process up to a million reactions a year. This guarantees the kit’s ability to fulfill significant demand and make a valuable contribution to the tracking and management of monkeypox outbreaks.
This RT-PCR kit’s capacity to test quickly is one of its main benefits. Its 40-minute turnaround time is a big improvement over traditional procedures, which usually take an hour or two. This quicker turnaround time is essential for more effectively managing public health responses, as it allows for quicker identification and intervention—both of which can be critical in halting the virus’s spread.
The National Institute of Virology (ICMR-NIV) of the Indian Council of Medical Research-Pune has clinically validated the IMDX Monkeypox Detection RT-PCR Assay. The kit’s remarkable 100% sensitivity and specificity are confirmed by the validation. This indicates that the kit is very accurate and that there are neither false positives nor false negatives, guaranteeing accurate viral detection.
Siemens Healthineers Managing Director Hariharan Subramanian stressed the significance of this development in the fight against monkeypox. He emphasized the need for timely and correct diagnosis, particularly given the state of the world’s health today, and how the new assay kit is a proactive measure to combat the illness. It is believed that having access to such cutting-edge diagnostic equipment will improve response to outbreaks of monkeypox and eventually save lives.
Following a spike in cases, mostly in Africa, the World Health Organization (WHO) recently designated the M-pox outbreak a public health emergency of international concern as of August 14, 2024. Since 2022, there have been about 30 cases of monkeypox reported in India; the most recent case was reported in March 2024. It is anticipated that the new diagnostic kit’s clearance and implementation will increase India’s ability to address this public health issue more skillfully.
SOURCE :
THE PRINT